Endotoxin and pyrogen test service
Based on long lasting experience
Based on long lasting experience in endotoxin and pyrogen testing, Microcoat offers a set of proprietary methods and skilled scientific personnel for non-routine projects. Services are run as flexible customer-specified projects including the search for root causes, exploration of realization alternatives, development of product-specific adaptions and validation of newly established methods.
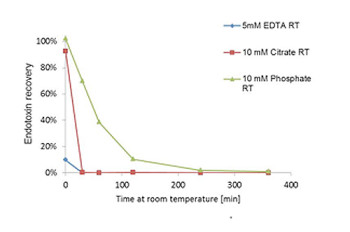
Low endotoxin recovery
LER (low endotoxin recovery) was first reported in 2013 (Chen et al. 2013) and describes the observation that endotoxin is underestimated due to masking in various drug product formulations with time. Reports exist where spiked endotoxin in drug formulations was not detectable by LAL but were pyrogenic in rabbit pyrogen test.
Typical formulations comprise a protein component, polysorbate, citrate or phosphate. The effect is strongly dependend on storage temperature, duration of storage, nature of the API and source of spiked endotoxin.
A LER hold-time study performed in our lab includes a detailed project plan and report. The experimental setup will be aligned with your specific needs. The turnaround time depends on the duration of the experimental phase but we guaranty short processing time for preparation of the study and generation of the report (in general 4 weeks).
Requests from FDA for biologics
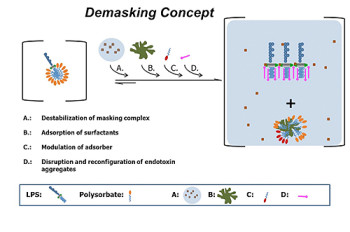
Demasking studies
As reported by the FDA, classical endotoxin test methods (LAL) can fail to detect masked endotoxin in various drug formulations (low endotoxin recovery).
In 2013 Hyglos GmbH launched a sample preparation kit for the demasking of endotoxin in biopharmaceuticals, EndoRS(1). The new method is based on the combinatorial use of the kit components for the screening for the best demasking mixture. The kit has to be applied in combination with EndoLISA detection method.
Removal of Endotoxin
Pyrogens produced by gram negative bacteria, i.e., the endotoxin (LPS), are of significance to the pharmaceutical industry (1). Endotoxin ingress is not only due to contaminations. Endotoxin can also occur as a result of the biotechnological process (e.g., E.coli expression system). Especially for such processes, dedicated endotoxin removal steps need to be clearly identified and validated. Thereby, endotoxin removal procedures can be challenging as high and also low levels of endotoxin need to be removed and the product shall remain without alteration.
Routine sample measurement
Endotoxin and pyrogen testing is mandatory during process development, as in-process control and for product release in pharmaceutical and medical device industry. In research (cell culture experiments and animal trails) contaminating endotoxins and other pyrogenic substances induce severe physiological reactions which often lead to false interpretation.
Microcoat has established a variety of commercial assays in order to narrow down the true nature of a contamination in a given sample. This may help to avoid false positive results and to provide improved strategies in process development and trouble shooting (OOS analysis). For differential testing two or more of the following assays may be applied:
Assay formats
Endotoxin detection
Pyrogen detection
Services
LAL assay
The kinetic LAL reagent is based on a cell lysate generated from the blood of the horseshoe crab, Limulus polyphemus. The lysate is containing a blood clotting enzyme cascade which is specifically activated by the interaction of endotoxin with Factor C. The activated Factor C activates Factor B and Factor B activates proclotting enzyme. The activated proclotting enzyme releases a dye from a chromogenic peptide substrate. Proclotting enzyme is also activated by ß-Glucan via the specific receptor Factor G.
Specifications
Advantages
Limitations
Regulatory status
Recombinant Factor C assay
Homogeneous in vitro assay. The assay reagent is containing a recombinant Factor C from horseshoe crab which becomes specifically activated by endotoxin. Activated Factor C is converting a peptide substrate and thereby releasing a fluorescent dye.
If not differently requested, the recombinant Factor C assay (EndoZyme®) from Hyglos GmbH is used for analysis.
Specifications
Advantages
Limitations
Regulatory status
EndoLISA®
Specific endotoxin test based on ELISA principle. Endotoxin is bound to a microwell plate by a LPS-specific phage protein. After washing off the sample matrix, bound LPS is detected using a recombinant Factor C and a chromogenic peptide substrate. EndoLISA® from Hyglos GmbH is used for analysis.
Specifications
Advantages
Limitations
Regulatory status
Monocyte activation test
The Monocyte acitivation test (MAT) is a cell-based assay: Toll-like receptor mediated stimulation of blood monocytes to produce pro-inflammatory cytokines in response to various pyrogens. The level of released cytokine is determined by a classical ELISA.
Monocyte Activation Test from different vendors are used:
Specifications
Advantages
Limitations
Regulatory status
MAT Proficiency Test Program
We offer quality assurance to laboratories working with Monocyte Activation Test (MAT). Based on our expertise we provide frequently audits of MAT test proficiency. Our Proficiency Test Program (PTP) confidentially verifies working procedures, method and report validation, identifies trends and potential demands for training. This supplies additional value to internal quality assurance.
Benefits
- Check and validate analysis techniques
- Verify conformity of procedures to requirements of the Pharmacopoeia
- Control and correct possible drift in procedural compliance
- Verify laboratory’s ability to recover correct level of contamination
- Compare results to those of other laboratories and other MAT methods
The 13th PTP trial is now open for registration
After submitting the results to ptp@microcoat.de, the final report will be distributed by Email.
Timeline for the 13th PTP trial:
Purpose
Participation
Process
Reporting
Get Started Today
ß-Glucan assay
This assay uses a specially treated limulus amebocyte lysate (LAL) where the Factor C stimulation pathway is inhibited. Therefore only activation by ß-glucan via Factor G leads to activation of proclotting enzyme and conversion of the chromogenic substrate.
The ß-glucan assay from Associates of Cape Cod (Glucatell®) is used for analysis.
Specifications
Advantages
Limitations
Regulatory status
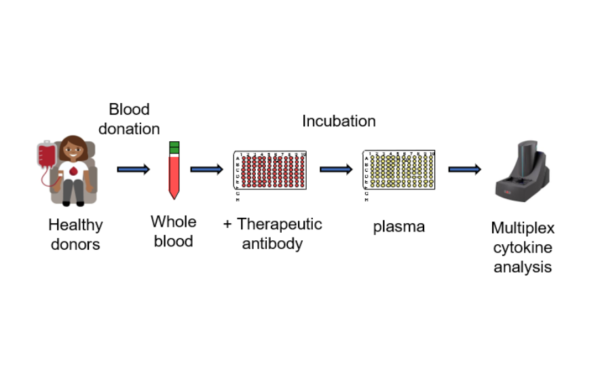
Whole blood assay
Novel therapeutic antibodies can cause severe responses and ultimately lead to life-threatening systemic release of cytokines – termed cytokine release syndrome (CRS) – as seen in a Phase I clinical trial following the administration of the CD28 superagonist antibody TGN1412.1 This demonstrates that (although in rare cases), infusion related responses in patients may only become apparent in first-in-man trials.
In 2020, the FDA released its Nonclinical Safety Evaluation of the Immunotoxic Potential of Drugs and Biologics emphasizing the use of “human cells before initiating clinical trials” as an “an assessment of the potential for cytokine release syndrome caused by therapeutic proteins using unstimulated human cells in both plate-bound (or other assays that can assess the contribution of crosslinking of receptors) and soluble formats with appropriate positive and negative controls”.
Microcoat’s Cytokine Release Assay
- Standardized
- Controls included
- Cohort of 30 healthy donors
Consulting
Microcoat provides consulting service in the field of pyrogen and bacterial endotoxin testing. Our team is specialized in the implementation of alternative test methods and provides advice in the case of test interference. For the analysis of the Low Endotoxin Recovery (LER) phenomenon, we provide dedicated guidance in planning, performing and interpreting of hold-time studies. Always giving you advice and quick support if you are facing unexpected trouble during execution of your studies. Furthermore, we support you in the development of strategies for mitigation of LER-affected products according to regulatory requirements.