Microcoat announces the 12th Monocyte-Activation Test Proficiency Test Program

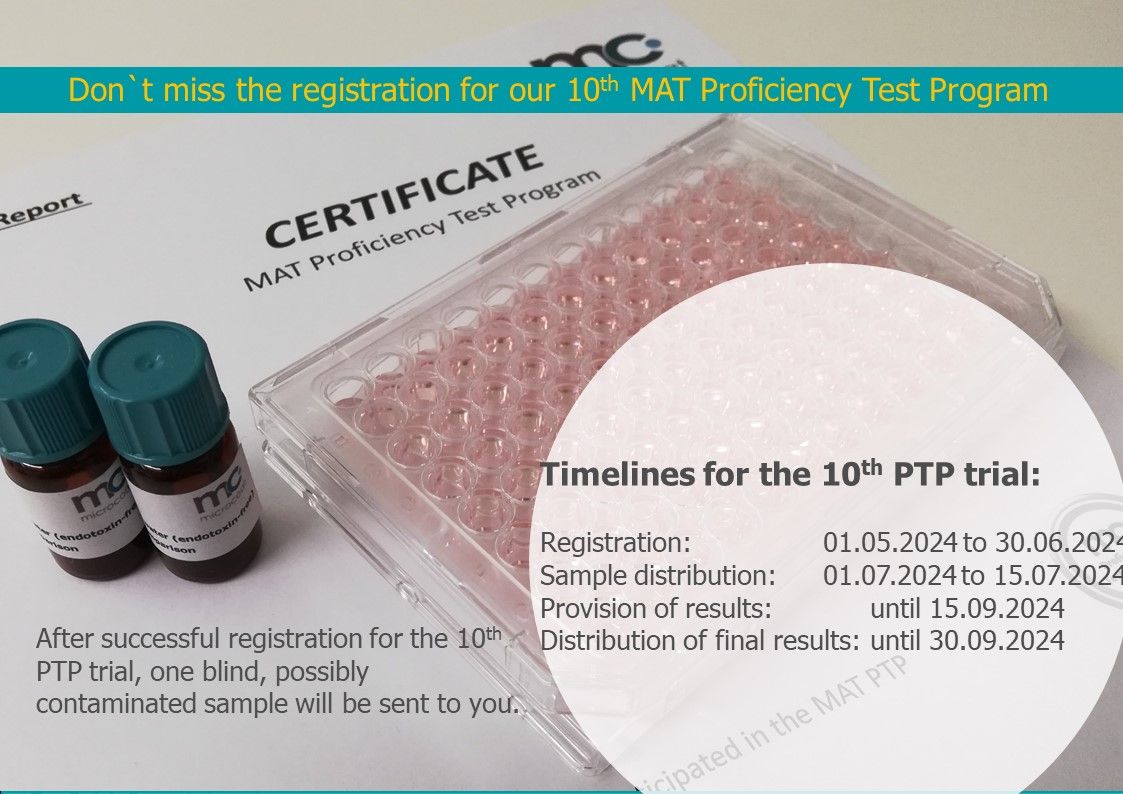
MAT Proficiency Test Program
We offer quality assurance to laboratories working with Monocyte Activation Test (MAT). Based on our expertise we provide frequently audits of MAT test proficiency. Our Proficiency Test Program (PTP) confidentially verifies working procedures, method and report validation, identifies trends and potential demands for training. This supplies additional value to internal quality assurance.
Benefits
- Check and validate analysis techniques
- Verify conformity of procedures to requirements of the Pharmacopoeia
- Control and correct possible drift in procedural compliance
- Verify laboratory’s ability to recover correct level of contamination
- Compare results to those of other laboratories and other MAT methods
MORE EVENTS
- 20März
Meet us at Analytica 2024
Read More → - 08Jan.
Low Endotoxin Recovery/Masking Training Course Munich/Bernried, GERMANY
Read More → - 09Feb.
Monocyte Activation Test (MAT) Munich/Bernried, GERMANY
Read More → - 09Feb.
Low Endotoxin Recovery/Masking Training Course Munich/Bernried, GERMANY
Read More → - 17Okt.
Meet us at the PharmaLab 2022 Düsseldorf/Neuss, Germany
Read More → - 29Sep.
Meet us at the PDA 2022, Pharmaceutical Microbiology Conference in Washington DC
Read More → - 20Sep.
Meet us at the Aseptikon 2022 Mannheim, Germany
Read More → - 07Okt.
Job- und Ausbildungsmesse 14.10.2021 11:00 Uhr bis 21:00 Uhr in der Tiefenstollenhalle Peißenberg
Read More → - 29Sep.
Job- und Ausbildungsmesse 05.10.2021 11:30 Uhr bis 21:00 Uhr in der Stadthalle Penzberg
Read More → - 14Mai
Digitale Jobmesse 2021 der Hochschule Biberach
Read More →